
Introduction
ActoHem is an active hemoglobin with high ability to bind oxygen and bring it to human and animal cells or cell cultures as needed. It is formulated as a solid-free solution with a low amount of methemoglobin and is suitable to serve as a potent oxygen carrier.
Goal
Our goal was to assess if gamma irradiation is a feasible and effective method for virus inactivation of ActoHem. Special attention was paid to the formation of methemoglobin or aggregates in the solution.
Methods
Hemoglobin- and Methemoglobin content was determined spectrophotometrically with a blood gas analyzer (ABL 725, 128-wavelength spectrophotometer, Radiometer GmbH). The content of solids/aggregates in the solution was evaluated with a 1:10 diluted sample by analyzing the size distribution (dynamic light scattering, Zeta-Sizer ZS nano, Malvern Panalytical GmbH). Gamma irradiation was performed in frozen condition by Mediscan GmbH & Co KG, Austria (www.mediscan.at) in compliance with EN ISO 13485 and ISO 11137 with a dose of 25 kGy (min – max: 26.5 – 33.6 kGy) and 35 kGy (min – max: 37.3 – 43.0 kGy) respectively (http://www.mediscan.at/en/technology/x-ray-technologie/).
Prior to irradiation, a validation procedure took place. For the irradiation, we selected an X-Ray method where an Accelerator Rhodotron TT300 is used. The reason for this selection is that X-Ray irradiation does not increase the product temperature as much as during the Cobalt gamma irradiation.
Sample
Frozen samples of ActoHem were thawed and the content of hemoglobin and methemoglobin was analyzed. Then the sample was filled in two 500 mL (35 kGy) and two 100 mL (25 kGy) plastic bottles, respectively and all bottles were frozen (-30 °C). For every dose of irradiation, one bottle was sent in frozen state to Mediscan while one bottle remained in the freezer at Biophyll and served as a reference. Spiked virus samples were also irradiated in order to prove the efficiency of the irradiation method. After irradiation the samples were sent back to Biophyll and, as well as the reference bottles, thawed and analyzed.
Results
After thawing, both irradiated samples had a dark brown color while the references showed the normal dark red color of hemoglobin. This could be a first indication of hemoglobin oxidation during irradiation as a brown color is typical for an increased amount of methemoglobin. In the same time, the virus test results showed that the viruses have been efficiently deactivated.
Methemoglobin
All samples, irradiated and reference, had a hemoglobin content of 4.75 ± 0.5 g/dL. The values for the amount of methemoglobin are shown in Table 1.
|
Sample |
Methemoglobin [%]; mean and standard deviation |
|
|
25 kGy |
35 kGy |
|
|
Reference |
13.35 ± 0.25 |
12.3 ± 0.9 |
|
Irradiated sample |
67.45 ± 0.15 |
74.7 ± 0.3 |
Table 1. Methemoglobin content in irradiated samples and untreated reference. Shown are the means with standard deviation.
In both irradiated samples the amount of methemoglobin increased massively. As methemoglobin is not able to transport or release oxygen, after irradiation only a small amount of hemoglobin remains functional.
Size distribution
The reference as well as the 25 kGy irradiated sample were analyzed by dynamic light scattering with a Zeta Sizer. The size distribution by intensity is shown in Figure 1.
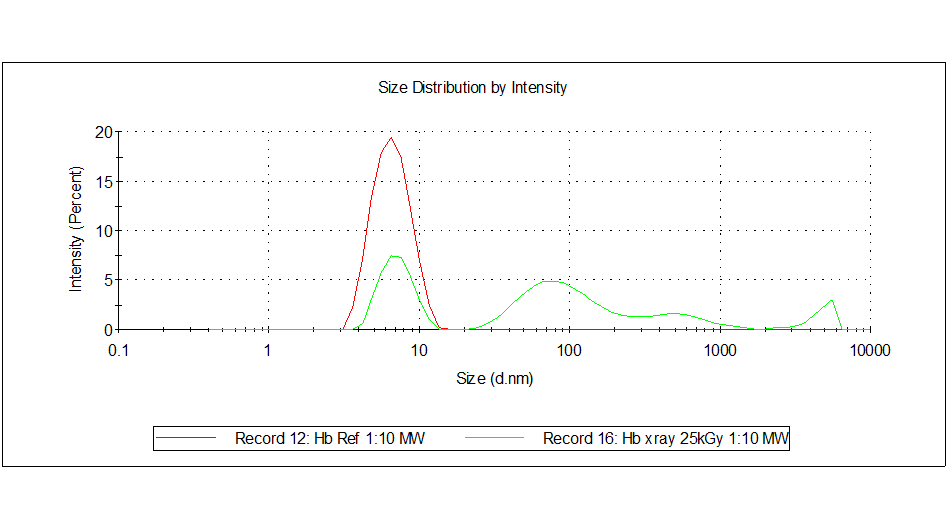
Figure 1. Size distribution by intensity. Red – reference, green – irradiated sample (25 kGy).
The reference sample exhibited only one peak at about 6.5 nm which is typical for hemoglobin. In the irradiated sample this peak was also present but there were additional peaks that indicated larger aggregates or particulate matter in a size range larger than 10 nm.
Conclusion
Gamma irradiation treatment leads to a high increase of the amount of methemoglobin. Methemoglobin is not able to transport oxygen and thus results in a loss of performance and ActoHem loses its key feature. Furthermore, there is particulate matter in the solution after irradiation maybe due to protein aggregation. Gamma irradiation is efficient for virus inactivation. However, the process cannot be recommended for virus inactivation in ActoHem while maintaining the capability of an effective oxygen transport.

